
Celerion Translates Science into Medicine
Welcome to the latest issue of the Celerion Science Newsletter, designed to provide to our readers information on the latest technologies, trends and stories specifically focused on early clinical development activities and professionals in clinical pharmacology and associated sciences. We invite you to share our vision, techniques and experiences learned through our accomplishments in all aspects of early clinical development.
This issue is focused on our bioanalytical capabilities. Celerion laboratories focus on science, compliance and speed to provide you with fast access to high-quality data. Our state-of-the-art automated facilities in Zürich, Switzerland and Lincoln, Nebraska USA, are among the most recognized laboratories in the industry. Celerion has over 40 years of experience working with large and small molecules, from discovery through late stage drug development. Through consistent enhancements of the facilities, Celerion provides rapid turnaround through completely automated sample analysis processes.
If there are any topics you would like to hear about, please email us at marketing@celerion.com.
Bioanalytical Excellence
Video: Biomarker Validation and Flow Cytometry: An Interview with Rafiq Islam

Rafiq Islam discusses the impact FDA guidance for industry on bioanalytical method validation has had on biomarker research in recent years. Rafiq also explains the advantages and disadvantages of flow cytometry as an analytical tool, before sharing his thoughts on technologies that will change biomarker research in the future.
Webcast: Challenges in Biosimilar Bioanalytical Assays and Sample Processing

The process of tackling biosimilar projects in bioanalytics can be challenging. René Wuttke, Bioanalytical Principal Investigator, Celerion, Zürich, Switzerland explores these challenges and defines experiments that should be conducted in method development and method validation in order to assess bioanalytical similarity and make optimal decisions on assay strategy.
White Paper: Qualification of an LC-MS/MS Method for the Simultaneous Determination of Desipramine and 2-Hydroxydesipramine in Human Plasma
Desipramine is a tricyclic antidepressant actively involved in blocking the reuptake of norepinephrine and serotonin within the brain. Desipramine can also be used to treat neuropathic pain associated with damage to the somatosensory nervous system.
White Paper: Recommendations for Classification of Commercial LBA Kits for Biomarkers in Drug Development from the GCC for Bioanalysis

Over the last decade, the use of biomarker data has become integral to drug development. Biomarkers are not only utilized for internal decision-making by sponsors; they are increasingly utilized to make critical decisions for drug safety and efficacy.
Case Study: Challenges and Solutions Associated with the Bioanalysis of Antibody Drug Conjugates in Support of Clinical Studies – A Case Study on Kadcyla®
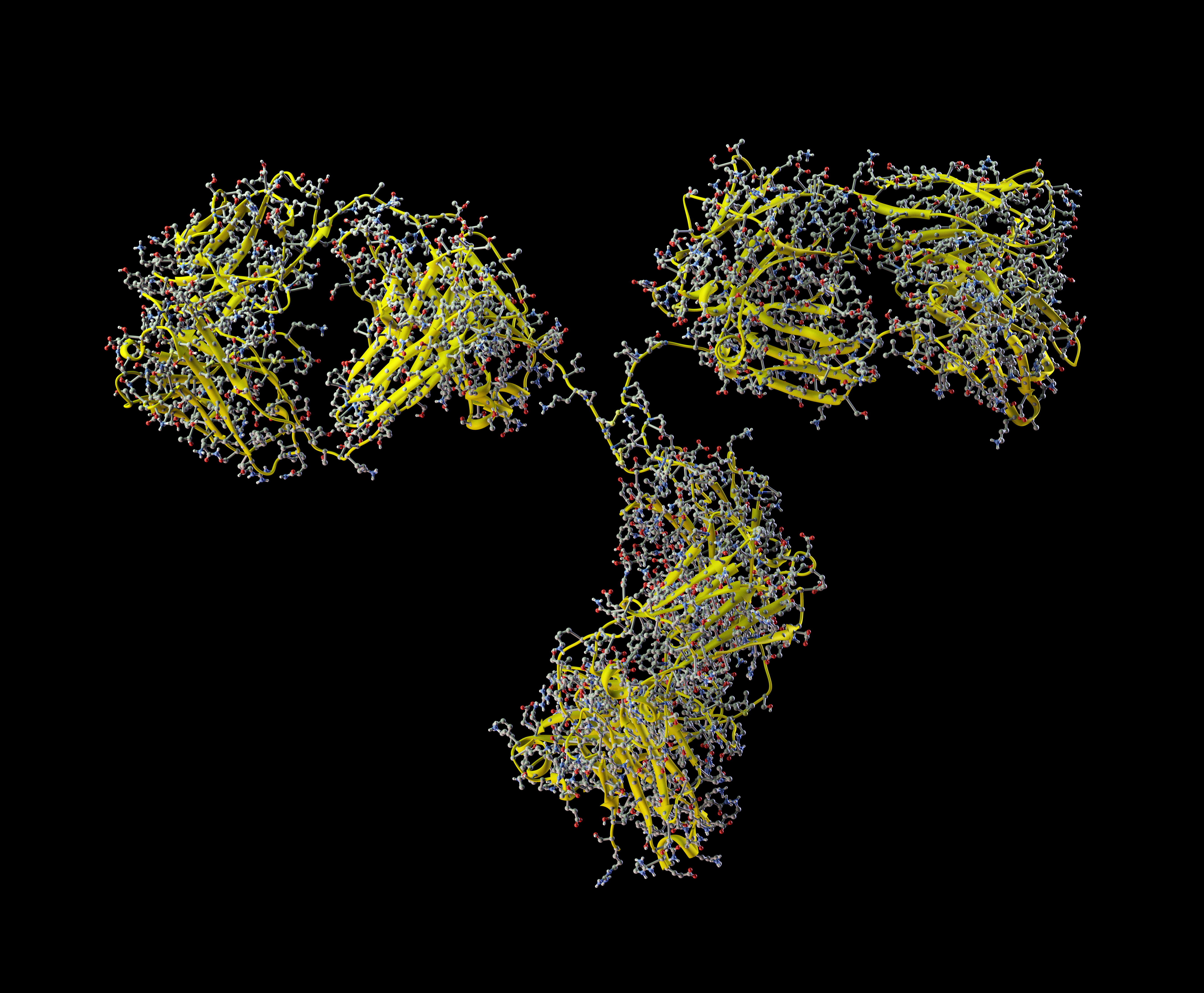
Antibody-drug Conjugates (ADCs) are one of the most innovative, and fast growing classes of potent bio-therapeutic drugs predominantly designed for targeted cancer therapy. ADCs are composed of a potent cytotoxic drug attached to a monoclonal antibody (mAb) via a linker.
Fact Sheet: Leaders in Biosimilars

Celerion has extensive experience and expertise in supporting biologics and biosimilar development programs.
Conference Poster Presentations
Physicochemical Interactions Between Analyte and Laboratory Material: Impact on Accuracy, YSS 2019 View Poster
A Novel Trap-and-Elute LC-MS/MS Method to Quantify Acyl Ghrelin and Des-Acyl Ghrelin in Human Plasma, WRIB 2019 View Poster
Validation of an IFN-g Elispot Assay in the Bioanalytical Laboratory, World Vaccine Congress 2019 View Poster
Immunogenicity Assessment of Pro- and Peptide Hormone Release in Engineered Tissues, EBF 2018 View Poster
A Sensitive and Robust Method for the Determination of Glucagon in Human Plasma by UHPLC-MS/MS, EBF 2018 View Poster
Alphalisa - A New, Simple, Highly Sensitive Technology for HTP Quantification of Small Human Proteins in Matrix, EBF 2018, View Poster
Biomarker Innovations
Scientific Poster: Challenges in FGF-21 Metabolic Biomarker Assay Development

Immune Fibroblast growth factor 21 (FGF-21) is a key peptide hormone involved in energy homeostasis.
Our People

Meet Rafiq Islam, Executive Director, Bioanalytical Sciences
Rafiq Islam is Executive Director of Bioanalytical Sciences at Celerion's Lincoln facility and responsible for the scientific and operational leadership of both small and large molecule bioanalysis, including bioanalytical and biomarker method development/ validation and execution of sample analysis to support pharmacokinetic, immunogenicity bioequivalence and biosimilar studies.
Previously, he served as the Scientific Director for Biopharma Services at EMD Millipore. He held similar positions as the head of a bioanalytical department at Covance and Huntingdon Life Sciences. He also held several positions of increasing responsibility with Curagen Corporation. He has nearly 20 years of biotechnology industry experience.

Meet Petra Struwe PhD, Executive Director, Bioanalytical Sciences
Petra Struwe is Executive Director of Bioanalytical Sciences at the Celerion facility in Zurich and responsible for the scientific and operational leadership for both small and large molecule bioanalysis. In this role she is responsible for bioanalytical, biomarker and immunogenicity method development, validation and associated sample analysis to support drug discovery, drug development and biosimilar research.
She holds a PhD in chemistry and before joining Celerion she held various positions with increasing responsibility in federal institutes, Solvay Pharmaceuticals and MDS Pharma Services. She has more than 20 years of industry experience in regulated bioanalysis within the pharmaceutical and CRO industry.
Events
|
BioFlorida: The Nuts and Bolts of Early Clinical Research 08.03.2019 | Alachua, FL
|
|
|
09.24.2019 - 09.25.2019 | San Diego, CA
|
|
|
10.02.2019 - 10.03.2019 | Boston, MA
|



